A common design challenge for medical device manufacturers is ensuring the filtration media can effectively prevent the spread of viral and bacterial particles in critical healthcare environments. Filtration media that is ineffective in this regard can lead to healthcare associated infections (HAI) or other viral agents which extend hospitalizations and costs for users of the device. In some cases, patient and healthcare workers’ lives can be at risk if there is not sufficient protection against blood-borne and aerosolized viruses.
From blood management to infusion filters, manufacturers can work with Oxyphen to determine the small particle retention and flow rates needed for their sterile filtration application. Our track-etched membrane technologies have highly defined pore structures to offer a safe and reliable solution for devices that must meet viral filtration efficiency (VFE) and bacterial filtration efficiency (BFE) standards. Oxyphen membranes have extremely high VFE (>99.9999%) and BFE (>99.9999%) ratings to preserve samples and reagents from contamination as well as protect workers from bloodborne pathogen exposure.

Highlighted Case Study

Electronic Textiles
Oxmotex AG is a research and development company dedicated to electroosmotic vapor and fluid transport, who developed revolutionary proprietary technology for electronically controlled moisture transport in membranes and textiles. As they looked to create a new innovative technology that would provide active electronically driven moisture transport, they came to Oxyphen for a track-etched membrane solution that would be responsive to changing climates, transport moisture beyond normal sweat rates, and be comfortable for the wearer.
Related Resources

Viral Filtration Efficiency (VFE) Certificate
Final test report from Nelson Labs for Viral Filtration Efficiency (VFE) at an Increased Challenge Level. Also available is the Bacterial Filtration Efficiency (BFE) report.

Bacterial Filtration Efficiency (BFE) Certificate
Final test report from Nelson Labs for Bacterial Filtration Efficiency (BFE) at an Increased Challenge Level. Also available is the Viral Filtration Efficiency (VFE) report.

Rollstock Membrane Product Brochure
Learn more about Oxyphen rollstock track-etched membrane material for in-house and large-scale manufacturing integration, which can be produced laminated or unlaminated, converted into different lengths, and surface treated upon request.
Register for our On-Demand Webinar
Delivering Precision & Control: An Introduction to Track-Etched Membrane Technology
Related Products

Rollstock Membrane
Ideally suited for in-house and large-scale manufacturing applications, Oxyphen’s track-etched rollstock membrane can be laminated, unlaminated, or fiber-based – supplied from polyester (PET) or polycarbonate (PC) high quality raw materials.

OxyPad® Self-Adhesive Pads
OxyPad® membrane pads, which are easily integrated inside or outside your housing design, promote consistent pressure equalization and protection against water, dust, and other harmful particulates.
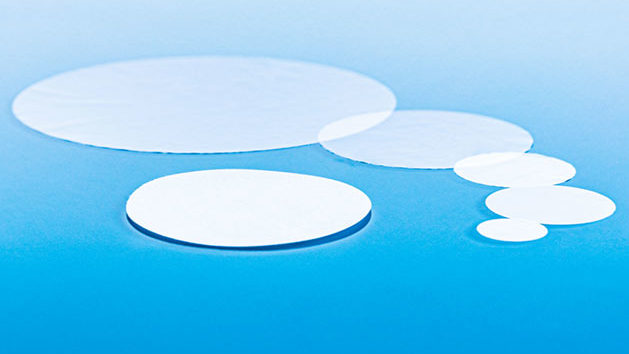
OxyDisc® Die-Cut Membrane Discs
OxyDisc® die-cut membrane discs can be easily mounted and produced using either hydrophobic or hydrophilic membranes for a variety of filtration or venting application requirements.
Membrane Technologies Available

Technologies
Unique-Mem® Unlaminated Track-Etched Membranes
Unique-Mem® track-etched membranes, available as hydrophilic or hydrophobic, are characterized by cylindrical shaped pores penetrating the membrane in different angles. They have a smooth flat surface and well-defined flow rates.

Technologies
RoTrac® Laminated Track-Etched Membranes
RoTrac® track-etched membranes are Unique-Mem® membranes that are supported with non-wovens (PP or PET) to create a more robust membrane. They are available as either hydrophilic or hydrophobic membranes.

Contact Us
Interested in speaking with one of our track-etched membrane experts?

